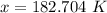Values of R, the ideal gas constant, and the ideal gas equation are given below. Use these to solve the problems.
PV = nRT
A 10.0-L rigid container holds 3.00 mol H2 gas at a pressure of 4.50 atm. What is the temperature of the gas? (Round to the nearest whole number)
K

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
Values of R, the ideal gas constant, and the ideal gas equation are given below. Use these to solve...
Questions



Mathematics, 05.05.2020 08:44



Mathematics, 05.05.2020 08:44

History, 05.05.2020 08:44

French, 05.05.2020 08:44

Mathematics, 05.05.2020 08:44

Business, 05.05.2020 08:44

Biology, 05.05.2020 08:44




Mathematics, 05.05.2020 08:44


Mathematics, 05.05.2020 08:44

History, 05.05.2020 08:44

Physics, 05.05.2020 08:45

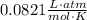 T is temperature (in Kelvins)
T is temperature (in Kelvins)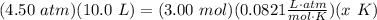 Isolate temperature x:
Isolate temperature x: 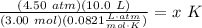 Rewrite:
Rewrite: 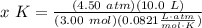 Evaluate:
Evaluate: