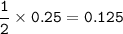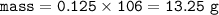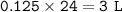Chemistry, 27.12.2020 03:10 makaylapink8167
3. The equation for the decomposition of sodium hydrogencarbonate is :
2 NaHCO3 (s) → Na2CO3 (s) + H2O(l) + CO2(8)
If 21 g of sodium hydrogencarbonate were decomposed, calculate
(a) the mass of the residue formed.
(b) the volume of carbon dioxide evolved measured at r. t.p.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3


Chemistry, 23.06.2019 18:10
Which property of a substance can be determined using a ph indicat
Answers: 3
You know the right answer?
3. The equation for the decomposition of sodium hydrogencarbonate is :
2 NaHCO3 (s) → Na2CO3 (s) +...
Questions

History, 13.01.2021 06:40


Mathematics, 13.01.2021 06:40

Chemistry, 13.01.2021 06:40

English, 13.01.2021 06:40

Mathematics, 13.01.2021 06:40

Mathematics, 13.01.2021 06:40





Physics, 13.01.2021 06:40

Mathematics, 13.01.2021 06:40


World Languages, 13.01.2021 06:40


Biology, 13.01.2021 06:40

Chemistry, 13.01.2021 06:40

Mathematics, 13.01.2021 06:40