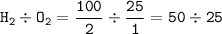Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
100cm^3 of hydrogen is mixed with 25cm^3 of oxygen at a temperature of 150*C. The gases react as sho...
Questions

Biology, 20.11.2020 14:00





Mathematics, 20.11.2020 14:00

Biology, 20.11.2020 14:00


English, 20.11.2020 14:00

Mathematics, 20.11.2020 14:00

Mathematics, 20.11.2020 14:00

Chemistry, 20.11.2020 14:00


Chemistry, 20.11.2020 14:00


Mathematics, 20.11.2020 14:00

Mathematics, 20.11.2020 14:00


Mathematics, 20.11.2020 14:00

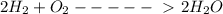 (All are gases)
(All are gases)