
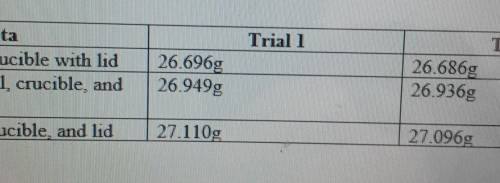
The actual yield of Magnesium Oxide in trial one is 0.414g, and in trial two it's 0.410.
With this information, what is the theoretical yield of MgO (in both trials) with Magnesium as the limiting reactant.
What is the percent yield of MgO for each trial.
What is the average percent yield of MgO for both trials.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
The actual yield of Magnesium Oxide in trial one is 0.414g, and in trial two it's 0.410.
With this...
Questions










Mathematics, 28.12.2019 04:31




Mathematics, 28.12.2019 04:31


Mathematics, 28.12.2019 04:31

Computers and Technology, 28.12.2019 04:31





