
Chemistry, 28.12.2020 08:00 Aliciaonfleek
A gaseous mixture of 1.0 mol of 12 and 1.0 mol of H2 was placed in an empty vessel that has a
volume of 2.0 L. The system was allowed to reach dynamic equilibrium at 300°C.
a) State briefly what happened to the initial concentration of 12 and H2 as the reaction reaches
equilibrium
b) Does the reaction stoop at equilibrium? Why?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
A gaseous mixture of 1.0 mol of 12 and 1.0 mol of H2 was placed in an empty vessel that has a
volum...
Questions






Biology, 15.07.2019 19:30


Mathematics, 15.07.2019 19:30







Biology, 15.07.2019 19:30


History, 15.07.2019 19:30

History, 15.07.2019 19:30


Biology, 15.07.2019 19:30

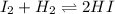
![\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/1008/4126/2a174.png)


