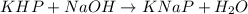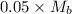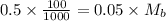Chemistry, 28.12.2020 15:50 zfghiiooi5
Someone help me please im stuck on a equation
3. Rearrange the titration calculation to find the Mb in a solution. (* Mb = molarity of the basic solution )
(~0.3 mL) of indicator to the KHP
solution.
(~ 0.5 mL or less) of NaOH
The moles of acid= 0.5 x 100/1000=0.05 Mb = (0.5/55)x1000=0.9

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

You know the right answer?
Someone help me please im stuck on a equation
3. Rearrange the titration calculation to find the Mb...
Questions



Computers and Technology, 28.09.2019 04:10







English, 28.09.2019 04:10

English, 28.09.2019 04:10


Business, 28.09.2019 04:10

Mathematics, 28.09.2019 04:10


Mathematics, 28.09.2019 04:10