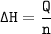Chemistry, 29.12.2020 01:00 mdakane3772
This table has information about the heat of fusion and the heat of vaporization of different substances. A 3-column table with 10 rows. Column 1 is labeled substance. Column 2 is labeled delta H fusion in kilojoules per mole. Column 3 is labeled delta H vaporization in kilojoules per mole. Ten rows are as follows: H 2 O, 6.01, 40.7. C O 2, 7.94, 25.2. O 2, 0.443, 6.81. N 2, 0.719, 5.58. F e, 14.9, 354. A l, 10.7, 255. C u, 13.0, 304. N a C l, 30.2, 171. C H 4, 0.936, 8.53. H 2 S, 2.37, 18.7. Which substance absorbs 58.16 kJ of energy when 3.11 mol vaporizes? Use q equals n delta H.. a. CH4 b. H2S c. CO2 d. NaCl

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1
You know the right answer?
This table has information about the heat of fusion and the heat of vaporization of different substa...
Questions

Mathematics, 12.12.2020 21:10



Biology, 12.12.2020 21:10

Mathematics, 12.12.2020 21:10

Computers and Technology, 12.12.2020 21:10

Biology, 12.12.2020 21:10

Mathematics, 12.12.2020 21:10

Mathematics, 12.12.2020 21:10



Mathematics, 12.12.2020 21:10

Mathematics, 12.12.2020 21:10




English, 12.12.2020 21:10

Arts, 12.12.2020 21:10


Biology, 12.12.2020 21:10