Chemistry, 30.12.2020 17:10 demarcuswiseman
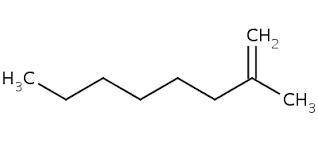
1) Compounds Y and Z both have the formula C9H18.
Both Y and Z react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methyloctane.
The heat of hydrogenation of Y is less than that of Z.
Y and Z each undergo hydroboration/oxidation to give a primary alcohol (OH attached to a primary carbon).
What is the structure of Y?
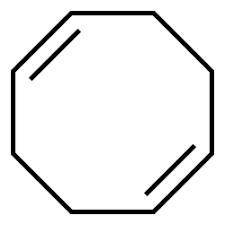
2) Compound X has the formula C8H12.
X reacts with two molar equivalents of hydrogen in the presence of a palladium catalyst to form cyclooctane.
Treatment of X with ozone followed by zinc in aqueous acid gives two molar equivalents of the same dialdehyde.
What is the structure of X

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
1) Compounds Y and Z both have the formula C9H18.
Both Y and Z react with one molar equivalent of h...
Questions


Computers and Technology, 02.01.2020 21:31







English, 02.01.2020 21:31


Social Studies, 02.01.2020 21:31

Computers and Technology, 02.01.2020 21:31

Social Studies, 02.01.2020 21:31