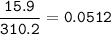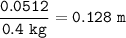Chemistry, 31.12.2020 02:30 kayleevilla
Show the calculation of the molality of a solution made by dissolving 15.9 grams of Ca3(PO4)2 in 400 grams of water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
Show the calculation of the molality of a solution made by dissolving 15.9 grams of Ca3(PO4)2 in 400...
Questions

Mathematics, 25.03.2021 19:10




Mathematics, 25.03.2021 19:10

Mathematics, 25.03.2021 19:10

Mathematics, 25.03.2021 19:10



History, 25.03.2021 19:10

Mathematics, 25.03.2021 19:10

Mathematics, 25.03.2021 19:10

Mathematics, 25.03.2021 19:10


Physics, 25.03.2021 19:10

Mathematics, 25.03.2021 19:10


Biology, 25.03.2021 19:10