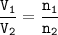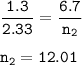Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

You know the right answer?
Two closed vessels contain chlorine gas at the same conditions of temperature and
pressure, so if t...
Questions



Mathematics, 27.10.2020 16:50

English, 27.10.2020 16:50

Chemistry, 27.10.2020 16:50


History, 27.10.2020 16:50

Chemistry, 27.10.2020 16:50


Mathematics, 27.10.2020 16:50



Mathematics, 27.10.2020 16:50

Chemistry, 27.10.2020 16:50

History, 27.10.2020 16:50

Mathematics, 27.10.2020 16:50

SAT, 27.10.2020 16:50

English, 27.10.2020 16:50

Physics, 27.10.2020 16:50

Mathematics, 27.10.2020 16:50