
PLEASE HELP WILL GIVE BRAINLEST
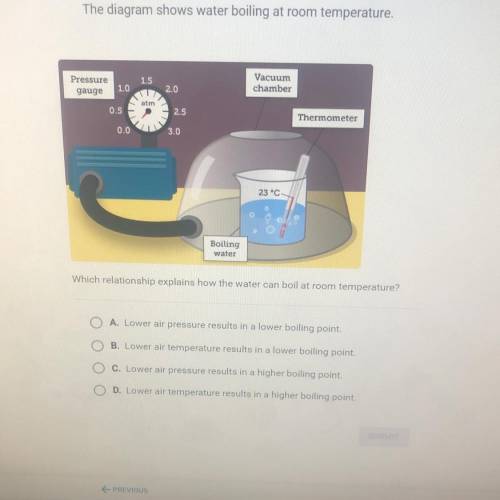
Which relationship explains how the water can boil at room temperature?
A Lower air pressure results in a lower boiling point
B. Lower air temperature results in a lower boiling point
C. Lower air pressure results in a higher boiling point
D. Lower at temperature results in a higher boiling point

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
PLEASE HELP WILL GIVE BRAINLEST
Which relationship explains how the water can boil at room temperat...
Questions

Mathematics, 14.11.2020 14:50


Law, 14.11.2020 14:50


English, 14.11.2020 14:50


Mathematics, 14.11.2020 14:50


Biology, 14.11.2020 14:50

Physics, 14.11.2020 14:50


English, 14.11.2020 15:00

Mathematics, 14.11.2020 15:00




History, 14.11.2020 15:00

Mathematics, 14.11.2020 15:00

Mathematics, 14.11.2020 15:00

English, 14.11.2020 15:00



