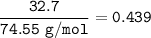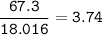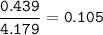An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in...

Chemistry, 31.12.2020 23:40 Chandler1Gaming
An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in
this aqueous solution?
Molar Mass
KCI: 74.55 g/mol
H2O: 18.016 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
Questions

History, 17.06.2020 10:57

Mathematics, 17.06.2020 10:57


Mathematics, 17.06.2020 10:57



Mathematics, 17.06.2020 10:57




Computers and Technology, 17.06.2020 10:57

Chemistry, 17.06.2020 10:57


Mathematics, 17.06.2020 10:57




Mathematics, 17.06.2020 10:57