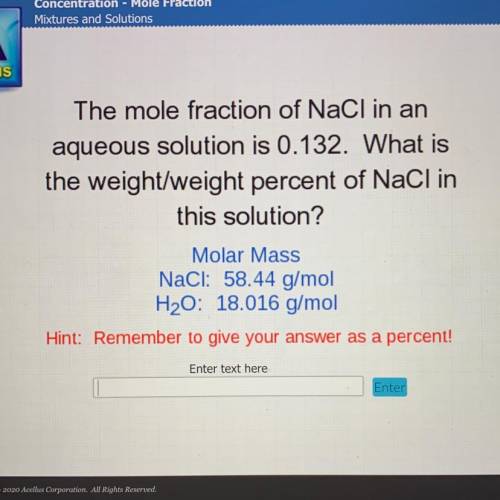
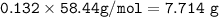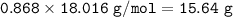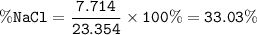The mole fraction of NaCl in an
aqueous solution is 0.132. What is
the weight/weight percent...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
Questions


Mathematics, 05.05.2021 09:00

Mathematics, 05.05.2021 09:00

History, 05.05.2021 09:00

Physics, 05.05.2021 09:00





Chemistry, 05.05.2021 09:00

Biology, 05.05.2021 09:00

Arts, 05.05.2021 09:00


Mathematics, 05.05.2021 09:00



Mathematics, 05.05.2021 09:00

History, 05.05.2021 09:00

Mathematics, 05.05.2021 09:00

Chemistry, 05.05.2021 09:00