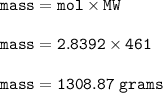Chemistry, 01.01.2021 14:00 irishvball7
18.2 mL of a 0.156 M solution of lead(II) nitrate are added to 26.2 mL of a 0.274 M solution of potassium iodide.
What is the mass of the Pbl2 precipitate formed in the reaction Pb(NO3)2 (aq) + 2 KI (aq) - Pbl2 (s) + 2 KNO3? The molar mass of Pbly is 461.0 g/mol.
Provide your answer in units of grams, with the correct number of significant digits. Enter your answer as a number only; do not include units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1


You know the right answer?
18.2 mL of a 0.156 M solution of lead(II) nitrate are added to 26.2 mL of a 0.274 M solution of pota...
Questions

Mathematics, 19.11.2020 07:40

Mathematics, 19.11.2020 07:40





Mathematics, 19.11.2020 07:40


History, 19.11.2020 07:40

Chemistry, 19.11.2020 07:40

World Languages, 19.11.2020 07:40

History, 19.11.2020 07:40




Mathematics, 19.11.2020 07:40


English, 19.11.2020 07:40


Mathematics, 19.11.2020 07:40