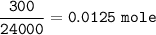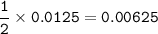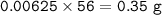4) 300cm of Hydrogen chloride gas were passed over 7.0g of heated iron fillings until there was no further
change. The reaction vessel was then allowed to cool to room temperature. Use the equation below to
determine the mass of iron that remained at the end of the experiment (molar gas volume = 24000cm"; Fe=56).
Fe(s) + 2HCl(g) → FeCl2(s) + H2(g)
(3 mks)
50
3 of IM colution calcium chloride (Avogadro's

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

You know the right answer?
4) 300cm of Hydrogen chloride gas were passed over 7.0g of heated iron fillings until there was no f...
Questions












Computers and Technology, 17.10.2019 04:10

Mathematics, 17.10.2019 04:10



Mathematics, 17.10.2019 04:10

Mathematics, 17.10.2019 04:10


German, 17.10.2019 04:10