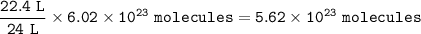Chemistry, 03.01.2021 01:00 jesuscruzm2020
You have two containers at 25°C and 1 atm. One has 22.4 L of hydrogen gas, and the other has 22.4 L of oxygen gas.
Which statement is true?
The hydrogen gas container has more molecules than the oxygen gas container.
The oxygen gas container has more molecules than the hydrogen gas container.
Both containers have the same number of molecules.
Both containers contain 6.022×1023 molecules of gas.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
You have two containers at 25°C and 1 atm. One has 22.4 L of hydrogen gas, and the other has 22.4 L...
Questions



Mathematics, 18.12.2020 21:00

Mathematics, 18.12.2020 21:00

Mathematics, 18.12.2020 21:00

Mathematics, 18.12.2020 21:00



Physics, 18.12.2020 21:00



Social Studies, 18.12.2020 21:00





Mathematics, 18.12.2020 21:00

Business, 18.12.2020 21:00


Business, 18.12.2020 21:00