
Chemistry, 03.01.2021 04:50 bossboybaker
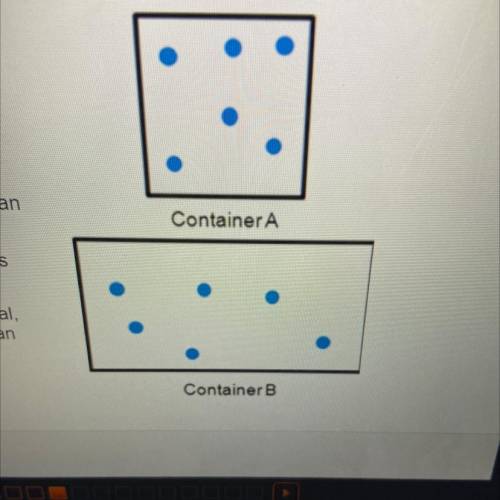
The diagrams to the right show the distribution
and arrangement of gas particles in two different
containers. According to kinetic-molecular theory,
which of the following statements is true? Check
all that apply.
If the temperatures of both containers are
equal, container A has greater pressure than
container B.
ContainerA
U
If the volume of container A decreased, its
pressure would decrease.
If the pressure in both containers is equal,
container A has a lower temperature than
container B.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
The diagrams to the right show the distribution
and arrangement of gas particles in two different
Questions

Mathematics, 02.12.2020 23:30


Mathematics, 02.12.2020 23:30


Mathematics, 02.12.2020 23:30



Mathematics, 02.12.2020 23:30

Mathematics, 02.12.2020 23:30

Biology, 02.12.2020 23:30

Mathematics, 02.12.2020 23:30

Chemistry, 02.12.2020 23:30


Mathematics, 02.12.2020 23:30

Mathematics, 02.12.2020 23:30

Physics, 02.12.2020 23:30






