Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Find the molar enthalpy of formation for paraffin wax (C2H526)) given the following reaction
C2H526...
Questions

Chemistry, 08.03.2021 07:30

Mathematics, 08.03.2021 07:30


Chemistry, 08.03.2021 07:30

Mathematics, 08.03.2021 07:30

Mathematics, 08.03.2021 07:30

Mathematics, 08.03.2021 07:30


Chemistry, 08.03.2021 07:30

Business, 08.03.2021 07:30


Mathematics, 08.03.2021 07:30



History, 08.03.2021 07:30

Social Studies, 08.03.2021 07:30

Chemistry, 08.03.2021 07:30


Chemistry, 08.03.2021 07:30

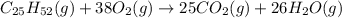
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/1012/0208/76c37.png)
![\Delta H=[(n_{CO_2}\times \Delta H_{CO_2})+(n_{H_2O}\times \Delta H_{H_2O})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{C_{25}H_{52}}\times \Delta H_{C_{25}H_{52}})]](/tpl/images/1012/0208/fc5e5.png)
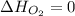 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![-14800=[(25\times -393.5)+(26\times -285.5)]-[(38\times 0)+(1\times \Delta H_{C_{25}H_{52}})]](/tpl/images/1012/0208/02342.png)