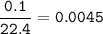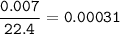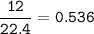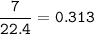Chemistry, 04.01.2021 20:30 Bassoonist
Which of the following gas samples would have the largest number of representative particles at STP?
a) 0.10 L Xe
b) 0.007 L SO3
c) 12.0 L He
d) 7.0 L O2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1
You know the right answer?
Which of the following gas samples would have the largest number of representative particles at STP?...
Questions

Mathematics, 02.08.2019 11:40


Mathematics, 02.08.2019 11:40



Chemistry, 02.08.2019 11:40

Health, 02.08.2019 11:40

Mathematics, 02.08.2019 11:40


Mathematics, 02.08.2019 11:40


History, 02.08.2019 11:50




Mathematics, 02.08.2019 11:50

Biology, 02.08.2019 11:50


Mathematics, 02.08.2019 11:50

English, 02.08.2019 11:50