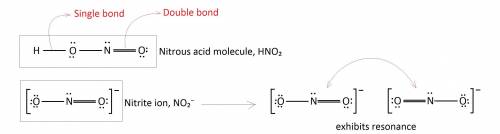
Incomplete Lewis structures for the nitrous acid molecule, HNO2, and the nitrite ion, NO2-, are shown here. (a) Complete each Lewis structure by adding electron pairs as needed. (b) Is the formal charge on N the same or different in these two species? (c) Would either HNO2 or NO2- be expected to exhibit resonance?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
Incomplete Lewis structures for the nitrous acid molecule, HNO2, and the nitrite ion, NO2-, are show...
Questions


English, 01.11.2019 06:31



Mathematics, 01.11.2019 06:31

Business, 01.11.2019 06:31



Mathematics, 01.11.2019 06:31