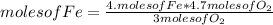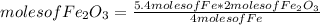Chemistry, 05.01.2021 16:30 gennhill14
If 5.4 moles of Fe react with 4.7 moles of O2, what is the maximum amount of Fe2O3 (in moles) that can be produced? What is the limiting reactant?
a
3.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
b
2.7 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.
c
7.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
d
10.8 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
If 5.4 moles of Fe react with 4.7 moles of O2, what is the maximum amount of Fe2O3 (in moles) that c...
Questions









Mathematics, 12.07.2021 22:10


Mathematics, 12.07.2021 22:10


English, 12.07.2021 22:10

English, 12.07.2021 22:10



Chemistry, 12.07.2021 22:10