
Chemistry, 05.01.2021 16:40 Skylar4483
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
of CO2?
2C2H2(g) + 502(g) → 2H2O(g) + 4CO2(g)
A. 20.0L
B. 44.8L
C. 80.0L
D. 100 L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
Questions

Mathematics, 09.09.2019 22:30


Social Studies, 09.09.2019 22:30




Advanced Placement (AP), 09.09.2019 22:30


History, 09.09.2019 22:30

Chemistry, 09.09.2019 22:30



Health, 09.09.2019 22:30

Mathematics, 09.09.2019 22:30

Social Studies, 09.09.2019 22:30




Physics, 09.09.2019 22:30

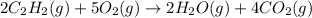
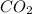 is formed by = 2 L of
is formed by = 2 L of 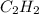
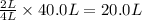 of
of 

