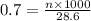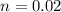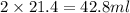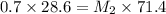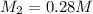Chemistry, 05.01.2021 16:50 highflylex279
You have a 0.7 M solution. Your job is to produce 50 mL of a 0.4 M solution.
A. How much of the 0.7 M solution do you need to start with? (Show your work.)
B. How many moles of solute were in the 0.7 M solution? (Show your work.)
C. How much water do you need to add to the previous amount of the 0.7 M solution to dilute it to
0.4 M? (Show your work.)
D. A student decides to put double the amount of water calculated in Part C. Describe what effect this
will have on the overall concentration of the resulting solution. Justify your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1


Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
You have a 0.7 M solution. Your job is to produce 50 mL of a 0.4 M solution.
A. How much of the 0.7...
Questions

Mathematics, 11.01.2022 22:50

French, 11.01.2022 22:50

German, 11.01.2022 22:50


Health, 11.01.2022 22:50

English, 11.01.2022 22:50






Business, 11.01.2022 22:50



Mathematics, 11.01.2022 23:00

Mathematics, 11.01.2022 23:00

Mathematics, 11.01.2022 23:00

History, 11.01.2022 23:00


Mathematics, 11.01.2022 23:00

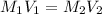
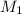 = molarity of stock solution = 0.7 M
= molarity of stock solution = 0.7 M
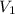 = volume of stock solution = ?
= volume of stock solution = ?
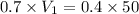
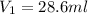
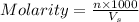
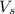 = volume of solution in ml
= volume of solution in ml