
Chemistry, 06.01.2021 01:40 eshavaggar
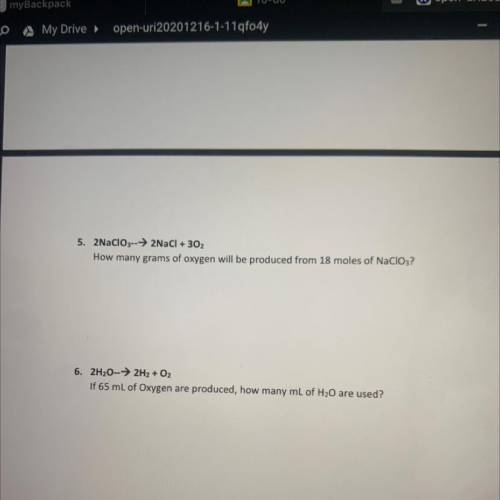
5. 2NaClO3-->2NaCl + 302
How many grams of oxygen will be produced from 18 moles of NaClO3?
6. 2H20-→ 2H2 + O2
If 65 mL of Oxygen are produced, how many mL of H20 are used?
Show all work

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
5. 2NaClO3-->2NaCl + 302
How many grams of oxygen will be produced from 18 moles of NaClO3?
Questions


Mathematics, 17.07.2019 22:30

Social Studies, 17.07.2019 22:30


Mathematics, 17.07.2019 22:30

Mathematics, 17.07.2019 22:30

Mathematics, 17.07.2019 22:30


Mathematics, 17.07.2019 22:30

English, 17.07.2019 22:30




Mathematics, 17.07.2019 22:30






Social Studies, 17.07.2019 22:30



