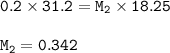Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Please help me A 0.200 M NaOH solution was used to titrate a 18.25 mL HE
solution. The endpoint was...
Questions


Physics, 02.09.2019 10:00









Computers and Technology, 02.09.2019 10:00

History, 02.09.2019 10:00


History, 02.09.2019 10:00

Physics, 02.09.2019 10:00


Chemistry, 02.09.2019 10:00


Mathematics, 02.09.2019 10:00