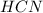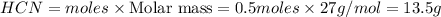Methane (CH4), ammonia (NH3), and oxygen (O2) can react to form hydrogen cyanide (HCN) and water according to this equation:
CH4+NH3+O2→HCN+H2O. A student has 8 g of methane and 10 g of ammonia in excess oxygen.
a. What is the balanced equation for this reaction?
b. Which reagent is limiting? Explain why.
c. How many grams of hydrogen cyanide will be formed?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
Methane (CH4), ammonia (NH3), and oxygen (O2) can react to form hydrogen cyanide (HCN) and water acc...
Questions




History, 14.04.2020 23:35



Mathematics, 14.04.2020 23:36

Mathematics, 14.04.2020 23:36



Mathematics, 14.04.2020 23:36

History, 14.04.2020 23:36






History, 14.04.2020 23:36


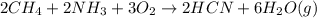
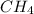 is the limiting.
is the limiting.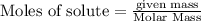
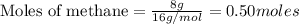
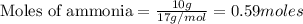
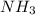
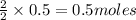 of
of