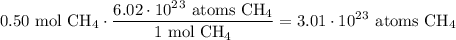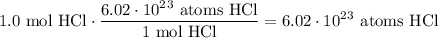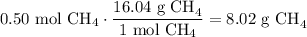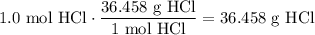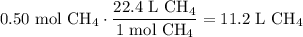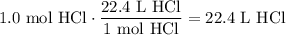Chemistry, 07.01.2021 14:00 truesarah111
Given the following two quantities: 0.50 mol of CH4 and 1.0 mol of HCl,
a. Which has more atoms?
b. Which has more molecules?
c. Which has the greater mass?
d. Which has the greater volume at the same temperature and pressure (both are gases)?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Given the following two quantities: 0.50 mol of CH4 and 1.0 mol of HCl,
a. Which has more atoms?
Questions


Computers and Technology, 17.07.2020 22:01

Mathematics, 17.07.2020 22:01




Mathematics, 17.07.2020 22:01


History, 17.07.2020 22:01

Mathematics, 17.07.2020 22:01

Mathematics, 17.07.2020 22:01



English, 17.07.2020 22:01

Mathematics, 17.07.2020 22:01