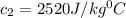A piece of glass has a temperature of 83˚C. Liquid that has a temperature of 43˚C is poured over the glass, completely covering it, and the temperature at equilibrium is 53˚C. The mass of liquid and glass is the same. If the specific heat of glass is 840 J/(kg˚C), determine the specific heat of the liquid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

You know the right answer?
A piece of glass has a temperature of 83˚C. Liquid that has a temperature of 43˚C is poured over the...
Questions

History, 26.03.2020 19:07

English, 26.03.2020 19:07

Mathematics, 26.03.2020 19:07

Mathematics, 26.03.2020 19:07

Physics, 26.03.2020 19:07

Physics, 26.03.2020 19:07






Mathematics, 26.03.2020 19:07





Geography, 26.03.2020 19:07

Mathematics, 26.03.2020 19:07


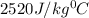
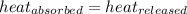
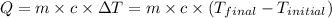
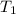 = temperature of glass =
= temperature of glass = 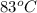
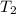 = temperature of liquid =
= temperature of liquid = 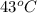
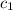 = specific heat of glass =
= specific heat of glass = 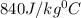
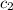 = specific heat of liquid= ?
= specific heat of liquid= ?![-[(x\times 840\times (53-83)]=x\times c_2\times (53-43)](/tpl/images/1018/8628/68a1f.png)