

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

You know the right answer?
What mass of solid aluminum hydroxide can be produced when 50.0mL of 0.200M aluminum nitrate is adde...
Questions

Health, 04.11.2019 02:31

Mathematics, 04.11.2019 02:31

Mathematics, 04.11.2019 02:31






Mathematics, 04.11.2019 02:31



History, 04.11.2019 02:31








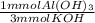 = 6.67 mmol Al(OH)₃
= 6.67 mmol Al(OH)₃

