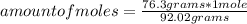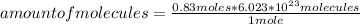Chemistry, 07.01.2021 18:30 orangeicecream
How many molecules of N2O4 are in 76.3g N2O4? The molar mass of N2O4 is 92.02 g/mol.
a. 4.59 × 10^25 N2O4 molecules
b. 5.54 × 10^25 N2O4 molecules
c. 7.26 × 10^23 N2O4 molecules
d. 1.38 × 10^24 N2O4 molecules
e. 4.99 × 10^23 N2O4 molecules

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

You know the right answer?
How many molecules of N2O4 are in 76.3g N2O4? The molar mass of N2O4 is 92.02 g/mol.
a. 4.59 × 10^2...
Questions

Physics, 30.05.2020 18:01


History, 30.05.2020 18:01


Mathematics, 30.05.2020 18:01

English, 30.05.2020 18:01


Mathematics, 30.05.2020 18:01

Spanish, 30.05.2020 18:01

Mathematics, 30.05.2020 18:01



Physics, 30.05.2020 18:01

Mathematics, 30.05.2020 18:01

History, 30.05.2020 18:01

Mathematics, 30.05.2020 18:01


Mathematics, 30.05.2020 18:02