
Chemistry, 07.01.2021 21:30 michellealvarez985
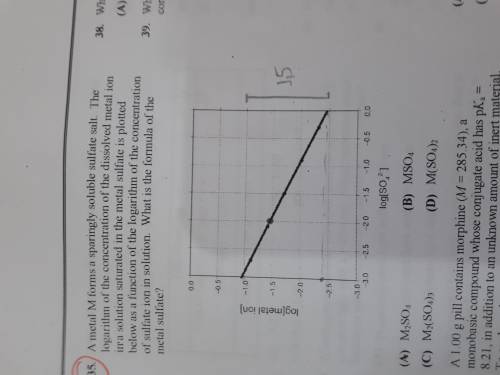
A metal M forms a sparingly soluble sulfate salt. The logarithm of the concentration of the dissolved metal ion in a solution saturated in the metal sulfate is plotted bellow as a function of the logarithm of the concentration of sulfate ion in solution. What is the formula of the metal sulfate?
a)M2SO4
b)MSO4
c)M2(SO4)3
d) M(SO4)2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2


Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
A metal M forms a sparingly soluble sulfate salt. The logarithm of the concentration of the dissolve...
Questions

Mathematics, 29.06.2019 06:20

Mathematics, 29.06.2019 06:20

Mathematics, 29.06.2019 06:20


Chemistry, 29.06.2019 06:20



Mathematics, 29.06.2019 06:20



Arts, 29.06.2019 06:20

Geography, 29.06.2019 06:20

History, 29.06.2019 06:20

History, 29.06.2019 06:20








