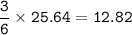Use the following reaction:
6 NaCl + Ba3(PO4)2 → 2 Na3PO4 + 3 BaCl2
How much barium ch...

Chemistry, 08.01.2021 01:00 talannajanis
Use the following reaction:
6 NaCl + Ba3(PO4)2 → 2 Na3PO4 + 3 BaCl2
How much barium chloride is produced (in g) when 1500 g of sodium chloride react with excess barium phosphate?
Round your answer to a whole number, do not write the units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Questions

Mathematics, 14.12.2020 21:40


Mathematics, 14.12.2020 21:40


English, 14.12.2020 21:40


English, 14.12.2020 21:40

Biology, 14.12.2020 21:40


Arts, 14.12.2020 21:40



Computers and Technology, 14.12.2020 21:40


English, 14.12.2020 21:40



Social Studies, 14.12.2020 21:40


Chemistry, 14.12.2020 21:40