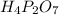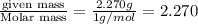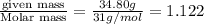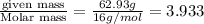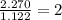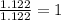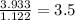Chemistry, 08.01.2021 02:20 goldenrizo
A compound is found to contain 2.270 % hydrogen, 34.80 % phosphorus, and 62.93 % oxygen by mass. What is the empirical formula for this compound

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
A compound is found to contain 2.270 % hydrogen, 34.80 % phosphorus, and 62.93 % oxygen by mass. Wha...
Questions

English, 04.12.2020 18:20

History, 04.12.2020 18:20


Computers and Technology, 04.12.2020 18:20


Mathematics, 04.12.2020 18:20

Mathematics, 04.12.2020 18:20


Physics, 04.12.2020 18:20


Geography, 04.12.2020 18:20

Chemistry, 04.12.2020 18:20


Mathematics, 04.12.2020 18:20


Arts, 04.12.2020 18:20




Mathematics, 04.12.2020 18:20