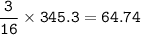Chemistry, 08.01.2021 06:10 sarahisme1123
One of the steps to sweeten sour gas using the Claus process is reacting
hydrogen sulfide gas with sulfur dioxide gas to produce water vapour and sulfur.
16 H2S(g) + 8 SO2(g) → 16 H2O(g) + 3 Sg(s)
8.56 kL of hydrogen sulfide at 175 kPa and 250 °C reacts with excess sulfur
dioxide. Calculate the mass, in kg, of sulfur produced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
One of the steps to sweeten sour gas using the Claus process is reacting
hydrogen sulfide gas with...
Questions

Mathematics, 03.11.2020 23:10

Mathematics, 03.11.2020 23:10


English, 03.11.2020 23:10


Advanced Placement (AP), 03.11.2020 23:10



History, 03.11.2020 23:10

Advanced Placement (AP), 03.11.2020 23:10

Physics, 03.11.2020 23:10

Mathematics, 03.11.2020 23:10



Mathematics, 03.11.2020 23:10

History, 03.11.2020 23:10


English, 03.11.2020 23:10