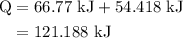Chemistry, 20.11.2019 19:31 Masielovebug
Heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( ) is heated to a final temperature of 65°c. determine the total amount of heat required to completely melt the sample. heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( ) is heated to a final temperature of 65°c. determine the total amount of heat required to completely melt the sample.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( )...
Questions



Biology, 18.12.2020 17:20




Mathematics, 18.12.2020 17:20

Chemistry, 18.12.2020 17:20






Mathematics, 18.12.2020 17:20





Medicine, 18.12.2020 17:20

Computers and Technology, 18.12.2020 17:20

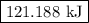 of energy is required to melt the sample completely.
of energy is required to melt the sample completely.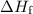 .
.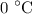 is converted to water at
is converted to water at 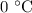 .
.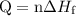 ...... (1)
...... (1) 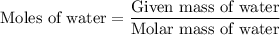 ...... (2)
...... (2) 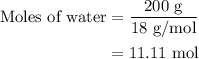
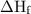 in equation (1).
in equation (1).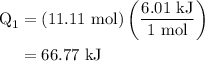
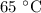 .
.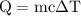 …… (3)
…… (3) 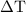 is the temperature change.
is the temperature change.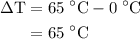
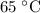 for
for 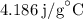 for c in equation (3).
for c in equation (3).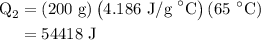
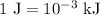
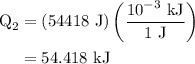
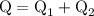 ...... (4)
...... (4) 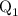 ,and
,and 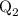 are the values of energies calculated in first and second step respectively.
are the values of energies calculated in first and second step respectively.