
Chemistry, 08.01.2021 17:40 baaaaaaaagoat6222
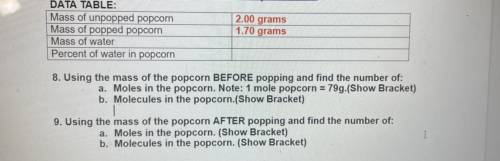
DATA TABLE:
Mass of unpopped popcorn
Mass of popped popcorn
Mass of water
Percent of water in popcorn
2.00 grams
1.70 grams
8. Using the mass of the popcorn BEFORE popping and find the number of:
a. Moles in the popcorn. Note: 1 mole popcorn = 79g.(Show Bracket)
b. Molecules in the popcorn.(Show Bracket)
9. Using the mass of the popcorn AFTER popping and find the number of:
a. Moles in the popcorn. (Show Bracket)
b. Molecules in the popcorn. (Show Bracket)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
DATA TABLE:
Mass of unpopped popcorn
Mass of popped popcorn
Mass of water
Percent...
Mass of popped popcorn
Mass of water
Percent...
Questions



History, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10

Computers and Technology, 21.01.2021 06:10

Social Studies, 21.01.2021 06:10

History, 21.01.2021 06:10

Social Studies, 21.01.2021 06:10

Biology, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10



Social Studies, 21.01.2021 06:10


Mathematics, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10

Mathematics, 21.01.2021 06:10



