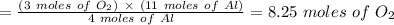Chemistry, 08.01.2021 18:40 maytce7237
Using the balanced chemical equation below. 2Al2O3 --> 4Al + 3O2 How many moles of oxygen are produced if 11.0 mol of Al are produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Using the balanced chemical equation below. 2Al2O3 --> 4Al + 3O2 How many moles of oxygen are pro...
Questions

Social Studies, 19.02.2021 09:00

Biology, 19.02.2021 09:00

Mathematics, 19.02.2021 09:00

Mathematics, 19.02.2021 09:00

Mathematics, 19.02.2021 09:00

Mathematics, 19.02.2021 09:00

Mathematics, 19.02.2021 09:00


Mathematics, 19.02.2021 09:00


Mathematics, 19.02.2021 09:00


Business, 19.02.2021 09:00



Mathematics, 19.02.2021 09:00

Mathematics, 19.02.2021 09:00

Social Studies, 19.02.2021 09:00