
Chemistry, 08.01.2021 20:10 kinderc9330
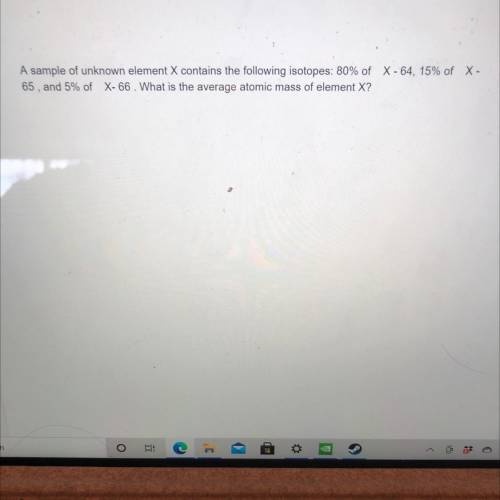
A sample of unknown element X contains the following isotopes: 80% of C -64, 15% of X - 65, and 5% of X-66. What is the average atomic mass of element X? Can somebody help me with this question. Will mark brainliest.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

You know the right answer?
A sample of unknown element X contains the following isotopes: 80% of C -64, 15% of X - 65, and 5% o...
Questions



Advanced Placement (AP), 20.02.2021 02:00

Mathematics, 20.02.2021 02:00

Biology, 20.02.2021 02:00

Mathematics, 20.02.2021 02:00

Mathematics, 20.02.2021 02:00

History, 20.02.2021 02:00







Mathematics, 20.02.2021 02:00

Biology, 20.02.2021 02:00

Mathematics, 20.02.2021 02:00

Mathematics, 20.02.2021 02:00

Mathematics, 20.02.2021 02:00

Mathematics, 20.02.2021 02:00



