
Chemistry, 09.01.2021 02:20 serenityjohnson98765
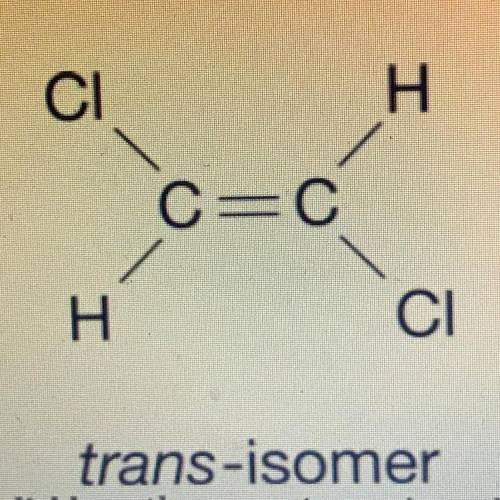
The structural formula for two isomers of 1, 2-dichloroethene are shown above. which of the two liquids has the higher equilibrium vapor pressure at 20° C and why
a) the cis-omer because it only has dipole dipole and the trans-iomer has london dispersion forces
b) cis-omer because it has only ldf and trans-iomer has dipole dipole
c) trans-iomer because it has only dipole dipole and cis-omer has ldf
d) trans-iomer because it has only ldf and cis-omer has dipole dipole

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
The structural formula for two isomers of 1, 2-dichloroethene are shown above. which of the two liqu...
Questions

Mathematics, 15.01.2020 17:31


Mathematics, 15.01.2020 17:31



Mathematics, 15.01.2020 17:31

Biology, 15.01.2020 17:31


Mathematics, 15.01.2020 17:31

Chemistry, 15.01.2020 17:31

Mathematics, 15.01.2020 17:31


Mathematics, 15.01.2020 17:31

Chemistry, 15.01.2020 17:31





History, 15.01.2020 17:31



