
Chemistry, 10.01.2021 05:50 samafeggins2
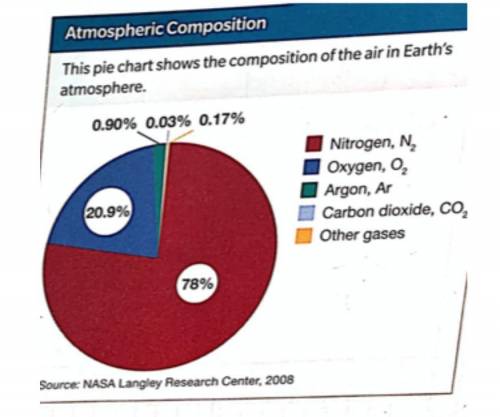
A compound is a pure substance made up of two or more different atoms joined by chemical bonds. Consider the composition of the air in Earth’s atmosphere. Which of the components of air is a compound? (Hint: refer back to your elements notes) How do you know?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2

You know the right answer?
A compound is a pure substance made up of two or more different atoms joined by chemical bonds. Cons...
Questions

Social Studies, 25.09.2019 05:30

Business, 25.09.2019 05:30

Health, 25.09.2019 05:30



Mathematics, 25.09.2019 05:30




Computers and Technology, 25.09.2019 05:30


Mathematics, 25.09.2019 05:30

Mathematics, 25.09.2019 05:30

Mathematics, 25.09.2019 05:30

Social Studies, 25.09.2019 05:30



Advanced Placement (AP), 25.09.2019 05:30


Mathematics, 25.09.2019 05:30



