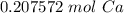How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are provided below. It is your responsibility to know which conversion factor to use!
Avogadro’s number: 6.02x1023 atoms = 1 mole
Molar mass of calcium: 40.078 g Ca = 1 mol Ca
A. 3.12x10^21 mol
B. 0.21 mol
C. 3.12 mol
D. 0.21x10^21 mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

You know the right answer?
How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are...
Questions

Advanced Placement (AP), 14.05.2021 01:00

History, 14.05.2021 01:00




Spanish, 14.05.2021 01:00


English, 14.05.2021 01:00

History, 14.05.2021 01:00


Chemistry, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00



Law, 14.05.2021 01:00

Chemistry, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00

Computers and Technology, 14.05.2021 01:00

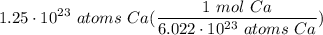 Multiply:
Multiply: