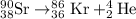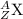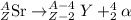Chemistry, 10.01.2021 08:50 kenziepickup
9. Write a balanced nuclear equation for the following: The isotope Strontium-90 decays by Q-
decay (1 point)
OfSr — He +56 kr
OS → H +39 kr
o Sr + Be +38kr
99 Sr → He +3 Se
SAST +7+99 Sr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
9. Write a balanced nuclear equation for the following: The isotope Strontium-90 decays by Q-
decay...
Questions

Mathematics, 11.05.2021 21:10


Mathematics, 11.05.2021 21:10


Mathematics, 11.05.2021 21:10



Mathematics, 11.05.2021 21:10


Mathematics, 11.05.2021 21:10

Chemistry, 11.05.2021 21:10


Mathematics, 11.05.2021 21:10

Mathematics, 11.05.2021 21:10




Mathematics, 11.05.2021 21:10


Mathematics, 11.05.2021 21:10