
Chemistry, 10.01.2021 22:20 Itsyourgirllulu
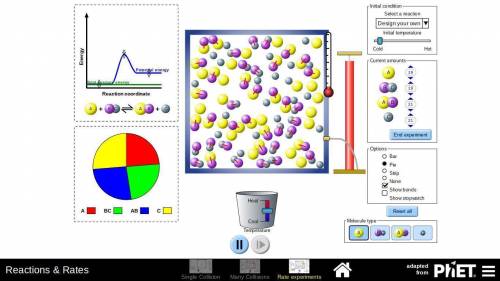
In part D of task 1, you identified at least two ways in which the reaction of nitrogen and hydrogen could be changed to alter the equilibrium. Use the simulation to test those changes. Describe how you used the simulation to model the changes and the results they produced. Use these methods if you find them helpful:
Look at the pie graph to see how the system changes.
Use the Temperature slider at the bottom to cool or heat the mixture.
Click the pause button on the simulation to observe the number of particles at any point of time.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1


Chemistry, 23.06.2019 09:30
People who practice which of the following diets may run the risk of not getting enough iron. a. gluten free or vegan diet b. diet for managing diabetes c. vegan diet d. gluten free diet
Answers: 2
You know the right answer?
In part D of task 1, you identified at least two ways in which the reaction of nitrogen and hydrogen...
Questions




History, 05.02.2020 04:57




English, 05.02.2020 04:57

History, 05.02.2020 04:57



Mathematics, 05.02.2020 04:57


Advanced Placement (AP), 05.02.2020 04:57




Biology, 05.02.2020 04:57

Mathematics, 05.02.2020 04:57

English, 05.02.2020 04:57



