
Chemistry, 11.01.2021 03:40 qveenjordan6456
Suppose 275 g of NO3- flows into a swamp each day. What volume of CO2 would be produced each day at 17.0°C and 1.00 atm? I attached the image of the equation for reference


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

You know the right answer?
Suppose 275 g of NO3- flows into a swamp each day. What volume of CO2 would be produced each day at...
Questions

Mathematics, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00



English, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00




Mathematics, 06.03.2021 01:00

Arts, 06.03.2021 01:00

History, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00




Geography, 06.03.2021 01:00

English, 06.03.2021 01:10

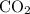 produced has been 264.28 L.
produced has been 264.28 L.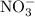 has been:
has been: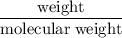
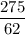
 Volume = moles
Volume = moles 

