
Chemistry, 11.01.2021 04:10 makennahudson94
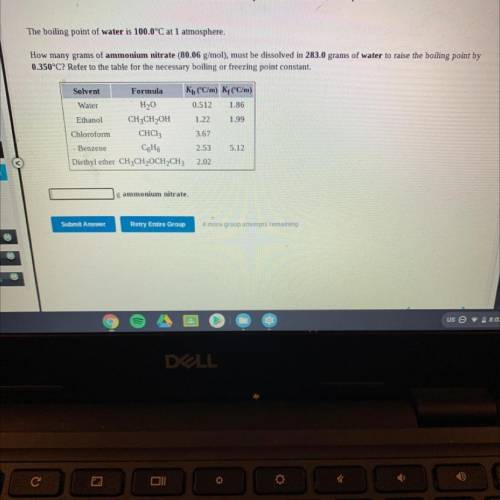
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/mol), must be dissolved in 283.0 grams of water to raise the boiling point by
0.350°C? Refer to the table for the necessary boiling or freezing point constant.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/...
Questions

Biology, 24.02.2021 20:30

English, 24.02.2021 20:30

Mathematics, 24.02.2021 20:30


Chemistry, 24.02.2021 20:30




Physics, 24.02.2021 20:30

Geography, 24.02.2021 20:30

Arts, 24.02.2021 20:30

Mathematics, 24.02.2021 20:30

Mathematics, 24.02.2021 20:30

Mathematics, 24.02.2021 20:30

Health, 24.02.2021 20:30



History, 24.02.2021 20:30




