Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
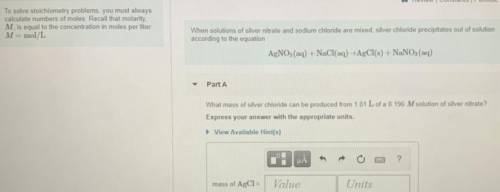
What mass of silver chloride can be produced from 1.01 L of a 0.196 M solution of silver nitrate?
E...
Questions



Mathematics, 11.06.2021 16:50




Mathematics, 11.06.2021 16:50



Social Studies, 11.06.2021 16:50


Social Studies, 11.06.2021 16:50

Mathematics, 11.06.2021 16:50

Social Studies, 11.06.2021 16:50




Mathematics, 11.06.2021 16:50

Mathematics, 11.06.2021 16:50

Biology, 11.06.2021 16:50