Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 3

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
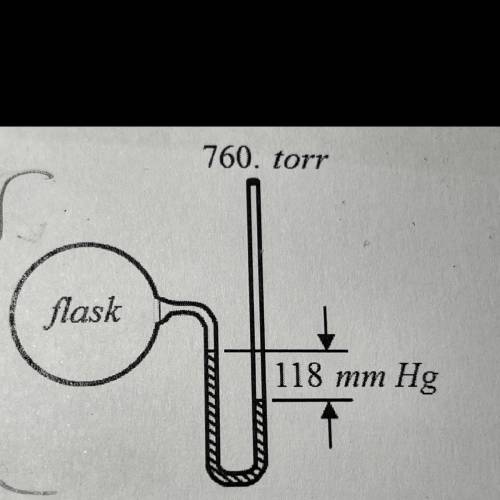
mercury’s density is 13.6 g/mL. now assume that the manometer shown above contains an oil density 1....
Questions

Mathematics, 09.12.2020 19:40





History, 09.12.2020 19:40

Mathematics, 09.12.2020 19:40


History, 09.12.2020 19:40




Social Studies, 09.12.2020 19:40


Physics, 09.12.2020 19:40


Mathematics, 09.12.2020 19:40