
Chemistry, 11.01.2021 15:20 morganluvsblueow9wtm
) Calculate the pH of a polyprotic acid given and sketch the titration curves for the following reactions: (a) A 20.0-mL aliquot of 0.100M arsenic acid, H3AsO4, with 0.100M NaOH

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2


Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
) Calculate the pH of a polyprotic acid given and sketch the titration curves for the following reac...
Questions


Health, 16.10.2020 01:01

History, 16.10.2020 01:01

English, 16.10.2020 01:01

Mathematics, 16.10.2020 01:01

History, 16.10.2020 01:01

Mathematics, 16.10.2020 01:01


History, 16.10.2020 01:01

English, 16.10.2020 01:01

Advanced Placement (AP), 16.10.2020 01:01

History, 16.10.2020 01:01


History, 16.10.2020 01:01

Mathematics, 16.10.2020 01:01

Mathematics, 16.10.2020 01:01



Mathematics, 16.10.2020 01:01

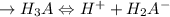
![\to Ka_1 = \frac{[H^+][H_2A^-]}{[H_3A]}](/tpl/images/1025/9491/6962a.png)
![= 10-2.2 = 6.31 \times 10^{-3} \ \ \ \ \ \ \ \ [since\ pKa_1 = 2.2\ and \ Ka = -\log_{10}(pKa)]\\](/tpl/images/1025/9491/eec45.png)
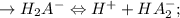
![\to Ka_2 = \frac{[H^+][HA_2^-]}{[H_2A^-]} = 10-6.8 = 1.58 \times 10^{-7} \\(since pKa2 = 6.8)\\\\\to HA_2^- \Leftrightarrow H^+ + A_3^- ;\\\\ \to Ka_3 = \frac{[H^+][A_3^-]}{[HA_2^-]} = 10-11.6 = 2.51 \times 10^{-12} \\ (given pKa_3 = 11.6)](/tpl/images/1025/9491/119c9.png)
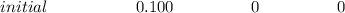
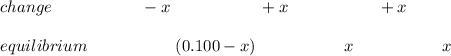
![Ka_1 = \frac{[H^+][H_2A^-]}{[H_3A]} = \frac{(x)(x)}{(0.100 - x)}](/tpl/images/1025/9491/3211c.png)
![\text{x to be much smaller than 0.100 M}\\\\to 6.31 \times 10^{-3} = \frac{x^2}{0.100}\\\\\to x^2 = 6.31 \times 10^{-4}\\\\ \to x = 0.025\ M\\\\\to pH = -\log_{10}[H^+] = -\log_{10}(0.025) = 1.60](/tpl/images/1025/9491/7bd66.png)


