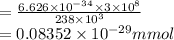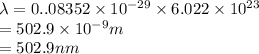Chemistry, 11.01.2021 15:40 vanessacox45
It takes to break a carbon-iodine single bond. Calculate the maximum wavelength of light for which a carbon-iodine single bond could be broken by absorbing a single photon.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
It takes to break a carbon-iodine single bond. Calculate the maximum wavelength of light for which a...
Questions




Biology, 16.09.2021 14:20

Mathematics, 16.09.2021 14:20

English, 16.09.2021 14:20

Physics, 16.09.2021 14:20


English, 16.09.2021 14:20


Mathematics, 16.09.2021 14:20

Mathematics, 16.09.2021 14:20

Mathematics, 16.09.2021 14:20

Biology, 16.09.2021 14:20


Mathematics, 16.09.2021 14:20

Chemistry, 16.09.2021 14:20


Computers and Technology, 16.09.2021 14:20

Chemistry, 16.09.2021 14:20