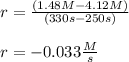Chemistry, 11.01.2021 15:50 asmith219771
Calculate the average rate of decomposition of NH4NO2 by the reaction 2NH4O5(aq) --> N2(g) H2O(g) at the following time interval. At time 250 s, the concentration of NH4NO2 is 4.12 M. At time 330 s, the concentration of NH4NO2 is 1.48 M

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
Calculate the average rate of decomposition of NH4NO2 by the reaction 2NH4O5(aq) --> N2(g) H2O(g)...
Questions




Mathematics, 25.09.2020 14:01



Chemistry, 25.09.2020 14:01


Geography, 25.09.2020 14:01


Mathematics, 25.09.2020 14:01



Physics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01



Mathematics, 25.09.2020 14:01

English, 25.09.2020 14:01

Business, 25.09.2020 14:01

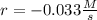
![r=\frac{\Delta [NH_4NO_2 ]}{\Delta t}](/tpl/images/1025/9856/b44bf.png)